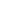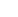80%
- Review and edit documents prepared by other writers (internal functional area representatives, CRO, or contractors) as required and ensure quality and adherence to standards.
- Independently prepare complex regulatory and clinical documents including protocols, Investigator’s Brochures (IBs), development safety update reports (DSURs), clinical study reports (CSRs), regulatory briefing documents, and clinical sections of regulatory submissions (IMPDs, INDs, CTAs, MAAs and BLAs).
- Oversight of CRO or consultant medical writers.
- Own the document development lifecycle by maintaining and driving document review cycles and timelines.
- Contribute to overall project management and cross functional working groups, as needed, to facilitate efficient development and finalization of documents for submissions.
- Ensure document adherence to standard operating procedures (SOPs), good clinical practice (GCP), and the International Council on Harmonization (ICH).
- Perform quality control of medical writing documents.
10%
- Assist in the development of templates, style guidelines, and SOPs.
10%
- Generate or assist in evaluating proposals, contracts, and change orders from CROs and other vendors.
Supervisory Responsibility:
This position has no supervisory responsibility.
Travel:
Travel may be required to visit the parent company, customers, factories, related organizations in China and elsewhere, about 15%.
Direct Report to: [Global CMO]
Work Environment:
The employee will perform the job in an office environment, or remotely.
Physical Demands:
- Extensive use of telephone and face-to-face communication requiring accurate perception of speech.
- Extensive use of keyboard requiring repetitive motion of fingers.
- Regular sitting for extended periods of time.
Required Technology:
Strong MS Excel, Word, and PowerPoint skills
Strong problem-solving skills and attention to details
Required Education and Experience:
- 8+ years clinical/regulatory writing experience with Master’s degree OR 6+ years clinical/regulatory writing experience with PhD in life science or equivalent
- Demonstrated ability to communicate and write clearly, concisely, and effectively
- Ability to complete tasks to deadlines and resolve/escalate problems in a timely manner under limited direction and on own initiative
- Experience with cross-functional study teams
- Proficient in Microsoft Word, Excel, Sharepoint or other joint writing/editing tools
- Strong analytical skills and ability to interpret and present complex data clearly
- Independently motivated, and good problem-solving ability
- Aptitude for compilation, analysis, and presentation of data
- Ability to work successfully within a cross-functional team and a matrix organization.
- Knowledge of current regulatory requirements and guidelines governing clinical research.
- Ability to manage and communicate effectively with vendors including reviewing requests for proposals, analyzing scope of work, and responding to inquiries and complaints.
- Must be able to work in a fast paced environment with demonstrated ability to manage multiple competing tasks and demands.
- Performs other work-related duties as assigned
Qualifications:
- Able to identify and resolve key issues.
- Highly accountable.
Other Duties:
Please note this job description is not designed to cover or contain a comprehensive listing of activities, duties or responsibilities that are required of the employee for this job. Duties, responsibilities and activities may change at any time with or without notice.
Contact E-mail:
Interested parties please send your resume to
HR email:lpswhr@lepubiopharma.com, HR@innocubebio.com
-
Principal Scientist, BiostatisticsU.S | 1 | PhD or equivalent degree in statistics/biostatistics or related discipline |
-
Director, Business DevelopmentU.S/China | 1 | Bachelor’s or equivalent degree in Biology or other life sciences majors is required |
-
Lead, Medical DirectorU.S | 1 | MD in oncology field |
-
Director, US Clinical OperationsU.S | 1 | Bachelor's degree in a health care or other scientific discipline or educational equivalent |