LEPU BIOPHARMA (2157.HK) is an innovation-driven biopharmaceutical company focusing on oncology therapeutics with a strong China root and global vision. On July 22, 2022 (Beijing time), the company announced that its first innovative biological drug, anti-PD-1 monoclonal antibody – Puyouheng (pucotenlimab injection), has been conditionally approved for marketing by the National Medical Products Administration (NMPA). The drug is applicable for patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) advanced solid tumors, which are as follows
● Patients with advanced colorectal cancers have progressed following previous treatment with a fluoropyrimidine, oxaliplatin and irinotecan;
● Patients with other advanced solid tumors that have progressed following at least previous first-line therapy with no satisfactory alternative treatment options.
The approval of Puyouheng (pucotenlimab injection) will bring a new option to tumor immunotherapies for more Chinese patients.
The approval is based on a multi-center, open-label, phase II clinical study with a primary study endpoint of the objective response rate (ORR) assessed by the Independent Review Committee (IRC) according to the RECIST1.1. As of December 4, 2021, a total of 100 patients with histologically confirmed advanced solid tumors that are identified as having MSI-H/dMMR by the central Laboratory were enrolled in the study. They were given 200 mg of pucotenlimab by intravenous drip every 3 weeks (Q3W). The median follow-up duration for the ITT population was 22.5 months. And the ORR for the ITT population was 49.0% (95% CI: 38.86%, 59.20%), with 9 cases of complete response and 40 cases of partial response. In the subgroup of patients with colorectal cancers who have failed previous triplet therapy (a fluoropyrimidine, oxaliplatin, and irinotecan), the ORR was 50.0% (95% CI: 31.30%, 68.70%). The results of the study showed that pucotenlimab monotherapy was safe and effective in patients with unresectable or metastatic MSI-H/dMMR advanced solid tumors who have failed previous standard therapy. The expected clinical endpoints were achieved, indicating that patients can benefit significantly from the therapy. The results of the clinical study were first published at the 2021 American Society of Clinical Oncology (ASCO) annual meeting.
About microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors
Microsatellite instability (MSI) refers to any changes in the microsatellite sequence caused by the insertion or deletion of repeat units in tumors compared with normal tissues, resulting in the appearance of new microsatellite alleles [1]. An impaired mismatch repair (MMR) system can cause these changes [2-3]. According to relevant literature, microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR) is observed in a variety of cancers, with an incidence of nearly 30% for endometrial carcinoma, approximately 20% for colon or gastric cancer, and less than 5% for other tumor types [4]. Due to greatly increased numbers of somatic mutations, MSI-H tumors express a large number of neoantigens, potentially rendering them more susceptible to immunotherapy than tumors with few mutations [4].
About Puyouheng (pucotenlimab injection)
Puyouheng (pucotenlimab injection) is a humanized IgG4 monoclonal antibody against human PD-1 independently developed in China. It can bind to PD-1 with high affinity to restore the ability of immune cells to kill cancer cells by blocking the binding of PD-1 to its ligands PD-L1 and PD-L2. Puyouheng (pucotenlimab injection) adopts an innovative molecular design to prolong its half-life, showing strong clinical anti-tumor activity and good safety. The innovative use of antibody engineering technology to introduce triple mutations in the Fc region improves the binding affinity of FcRn, thereby prolonging the drug’s half-life significantly, showing its promising clinical efficacy and drug compliance in patients. Compared with all rival anti-PD-1 antibodies that have been marketed or entered phase III clinical trials, the mean half-life of Puyouheng (pucotenlimab injection) is 21.8 days (single dosing) and 38.2 days (steady-state). In addition, the prolonged half-life does not cause additional adverse events, indicating the excellent clinical efficacy of the drug.
About the clinical research and development project of Puyouheng and its progress
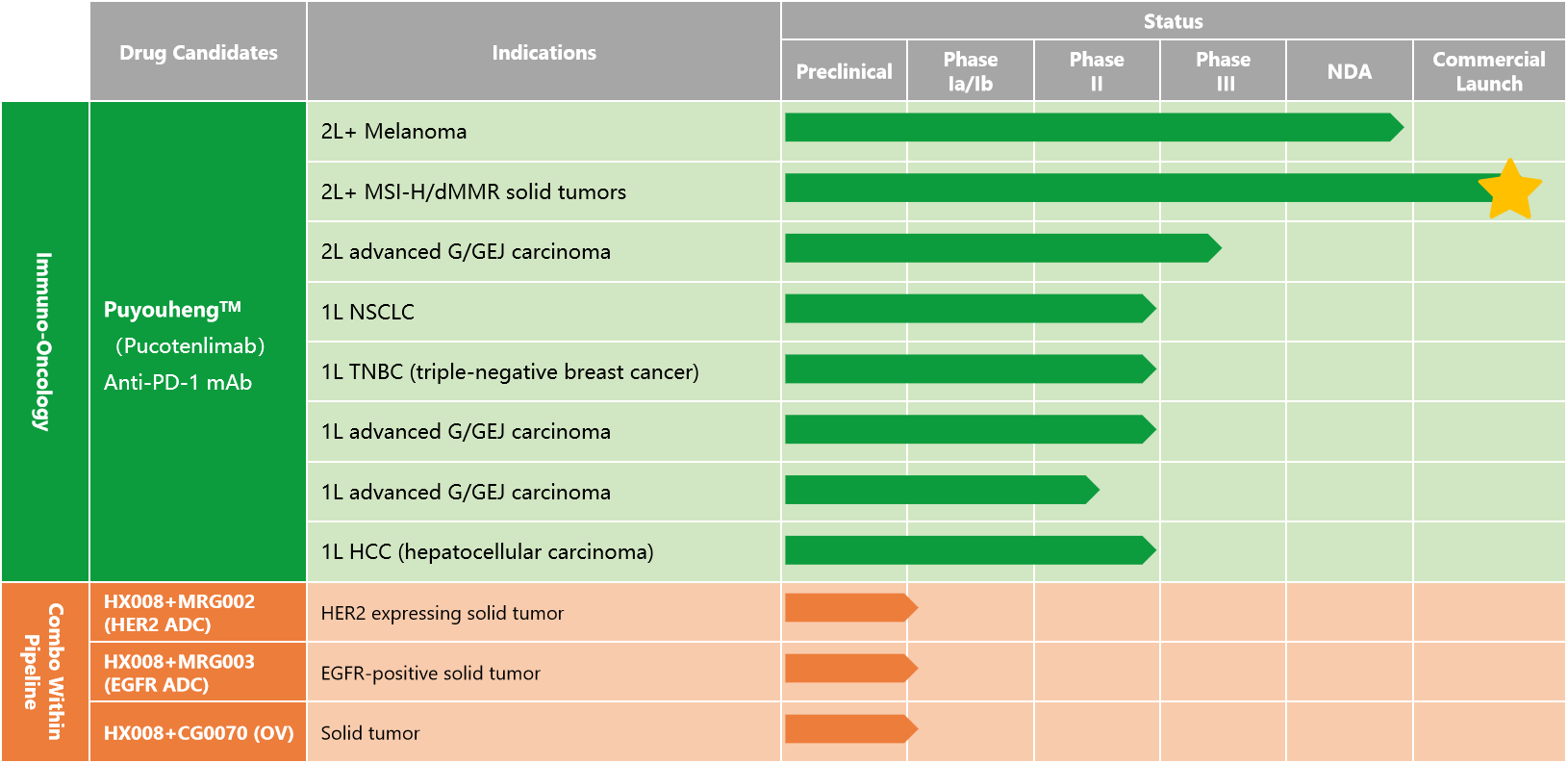
In addition to the approved indications of MSI-H/dMMR advanced solid tumors, the marketing application for the indication of Puyouheng (pucotenlimab injection) as a single drug for the second-line therapy of advanced melanoma has been accepted by NMPA in July 2021. Focusing on Puyouheng (pucotenlimab injection), LEPU BIOPHARMA is accelerating its development in the treatment of multiple solid tumors and actively investigating combined tumor immune immunotherapies, which has covered the first-line therapy for gastric cancer, liver cancer, lung cancer and other cancer types with high incidence. In terms of international development, LEPU BIOPHARMA is also speeding up its efforts to expand the international market, promoting joint development, cooperation and authorization of new drugs globally. In January 2022, it obtained IND approval from the US FDA for pucotenlimab in the treatment of advanced solid tumors.
About LEPU BIOPHARMA (2157.HK)
LEPU BIOPHARMA is committed to innovation, focusing on the discovery, development and commercialization of first-in-class and best-in-class drug candidates in targeted anti-tumor therapy and immunotherapy drugs in China and the US. The company’s mission is to develop the safest, most effective, and most accessible drugs for patients to improve their quality of life and fill the huge demand gap in the medical system. The company attaches great importance to the continuous construction of its commercialization capabilities, and strives to achieve a strong transformation from core technologies to finished drugs as well as the goal of industrialization. At present, the product pipeline of LEPU BIOPHARMA covers three major areas, namely immunotherapies, ADC targeted therapies and oncolytic virus drugs, including 1 commercially marketing drug and 7 drug candidates at the clinical stage (5 of which are ADC drugs) and the combination therapies of multiple major drug candidates at the clinical stage. The company houses the leading ADC drug candidate pipeline in China.
References:
[1] Gandhi JS, Goswami M, Sharma A, et al. Clinical impact of mismatch repair protein testing on outcome of early staged colorectal carcinomas[J]. J Gastrointest Cancer. 2017 Jun 5.
[2] Sclafani F. PD-1 inhibition in metastatic dMMR/MSI-H colorectal cancer[J]. Lancet Oncol.2017 Sep;18(9):1141-1142.
[3] Sargent DJ, Marsoni S, Monges G, T, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer[J]. J Clin Oncol. 2010 Jul 10;28(20):3219-26.
[4] Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors[J]. Clin Cancer Res. 2019;25(13):3753-3758.