The application for clinical trial of "CG0070 Injection" by Lepu Biopharma Co., Ltd. (hereinafter referred to as"Lepu Biopharma") has been accepted by Center for Drug Evaluation (CDE), NMPA (Acceptance No.: JXSL2100029) and publicized on March 4, 2021. CG0070 oncolytic virus antineoplastic drug is brought in by Lepu Biopharma fron CG Oncology, Inc., USA., obtaining its product development rights and global supply rights in China.
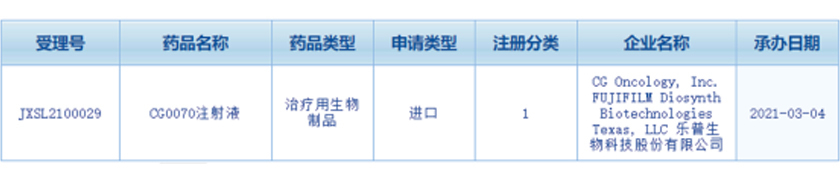
Image from CDE
Oncolytic viruses can selectively replicate in tumor cells and then lyse tumor cells, and could meanwhile try to avoid affecting the growth of normal cells; with the development of cancer immunotherapy, the therapeutic potential of oncolytic viruses in various diseases such as malignant tumors has received more and more attention. Since the US FDA approved Amgen's T-VEC oncolytic virus antineoplastic drugs in 2015, a number of oncolytic virus antineoplastic drugs have entered clinical studies worldwide. At the same time, a number of clinical studies have also been carried out on the synergistic effect of the combination of oncolytic viruses and other anti-tumor drugs.
CG0070, an oncolytic virus antitumor agent, is a genetically modified adenovirus type 5 (Ad5) that is modified to contain the cancer-selective promoter E2F-1 and the human granulocyte macrophage-colony stimulating factor GM-CSF gene to selectively replicate and lyse tumor cells in tumor cells with defective Rb regulation. Lysis of cancer cells releases tumor-specific antigens and GM-CSF expressed with the virus, which stimulates a systemic anti-tumor immune response.
In recent years, Lepu Biopharma has actively step into the field of oncolytic viruses. In addition to introducing the CG0070 project of CG Oncology, Inc., Lepu Biopharma has also taken a stake in Wuhan Binhui Biopharm Co., Ltd., and has initiated a clinical trial of the combination of immune checkpoint inhibitors and oncolysis virus (OH2)..